|
艾滋病2006:第16届国际艾滋病会议
AIDS 2006: XVI International AIDS Conference
2006年8月13-18日
加拿大安大略省多伦多
August 13 - 18, 2006, Toronto, Ontario, Canada
Antiretroviral Treatment Strategies for Adults
David Cooper, MD
Strategy trials with immediate clinical implications constituted the overarching theme of antiretroviral therapy trials presented at the International AIDS Conference (IAC) held in Toronto, Ontario, Canada, July 13-18, 2006. Continuing a theme that began at the Conference on Retroviruses and Opportunistic Infections (CROI) earlier this year, structured treatment interruptions were a focus of this conference as well. Other important trials examined the use of a boosted protease inhibitor to start or simplify antiretroviral therapy (ART), and other trials continued to examine the optimum combination of antiretroviral agents (ARVs) to use as initial therapy.
Structured Treatment Interruptions
Structured, or "strategic," treatment interruptions (STIs) offer the potential to spare patients some of the adverse effects, cumulative toxicity, and financial burden of ART. These are critical considerations, given that many patients might use antiretroviral drugs for decades after they start therapy. Therefore, it was reasonable to evaluate whether a treatment interruption strategy was associated with any changes in the rate of disease progression or toxicity. The SMART study was designed to address this question and enrolled over 5000 patients, making it the largest ART clinical trial ever conducted. Investigators randomized patients with CD4+ cell counts > 350/microliter (mcL) to either use continuous ART to maintain the viral load as low as possible (virologic suppression [VS] arm, n = 2752) or to take ART episodically (discontinuation [DC] arm, n = 2720). CD4+ cell counts were used as guides for starting and stopping therapy; ART was discontinued until the CD4+ cell count fell below 250, and then ART was resumed when the CD4+ cell count rose above 350. To the surprise of many, an interim analysis showed a statistically significant increase in clinical endpoints, including deaths, in the treatment interruption arm. The study was terminated prematurely, and the results were presented at CROI.[1] Of particular note, there was an elevated risk for major cardiovascular events, such as myocardial infarction, in the DC group.
Analyses continued to attempt to explain the reasons for the results, and these data were presented at IAC. Wafaa El-Sadr and colleagues[2] presented analyses that compared the hazard ratio in subgroups in the study arms in order to determine whether particular factors would provide insight into the reasons for the adverse outcomes in the DC arm, or whether there were subgroups of patients who benefited from the interruption in ART. Factors that were evaluated included:
· baseline HIV disease stage, including prior AIDS-defining condition, CD4+ cell count, CD4%, viral load, and coinfection;
· history of ART use, including number of ARVs used and duration of ART; and/or
· demographic characteristics such as race/ethnicity, sex, age, and history of intravenous-drug use.
The result of these analyses was that no subgroups were found in which the DC arm was superior to the VS arm. Two examples of the data from these analyses are shown in Figures 1 and 2. The conclusion was that STI, according to the SMART study design, "should not be recommended for any subgroup of patients."
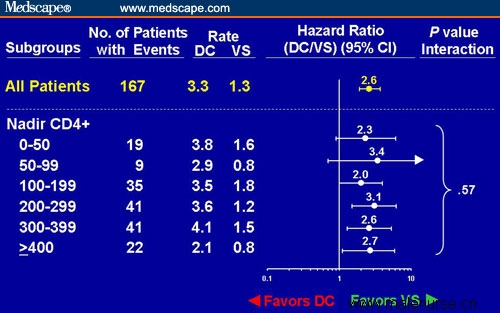
Figure 1. Risk for opportunistic disease or death in subjects in the SMART trial randomized to the DC vs VS study arms analyzed by nadir CD4+ cell count. Adapted from El-Sadr et al[2] with permission.
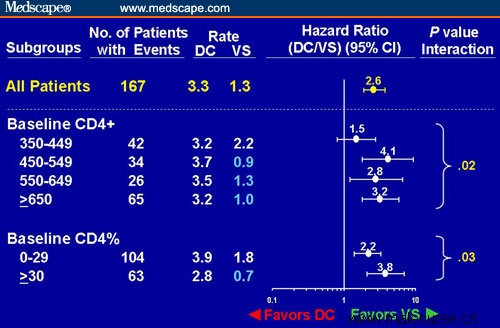
Figure 2. Risk for opportunistic disease or death in subjects in the SMART trial randomized to the DC vs VS study arms analyzed by baseline CD4+ cell count and CD4%. Adapted from El-Sadr et al[2] with permission.
During the treatment interruption, CD4+ cell counts fell and viral load increased. To what extent do these factors explain the results in the SMART study? According to the analyses presented by Lundgren and coinvestigators[3] for the SMART investigators, the CD4+ cell count (Figure 3) and viral load recorded proximal to the clinical event were associated with a greater risk for opportunistic disease (OD) and death. Together the latest CD4+ cell count and the viral load did explain part, but not all, of the increased risk for clinical events (Figure 3).
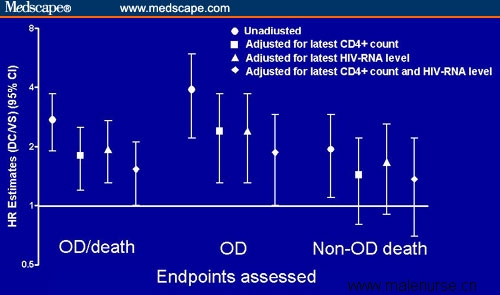
Figure 3. Comparison of adjusted and unadjusted hazard ratios (HR) for OD and death, OD, and non-OD death in the DC/VS study arms. Adapted from Lundgren et al[3] with permission.
The greatest increment in the incidence of clinical events was among the people who had the highest CD4+ cell counts (Figure 4), and it is those people who suffered the most damage from the DC strategy. Some researchers have hypothesized that there might be a massive inflammatory response resulting from virologic rebound after sustained virologic suppression, and such an inflammatory response might also explain the increased risk for heart attacks. Another analysis of the SMART data showed that the DC strategy did not improve the quality of life by a variety of measures, including general health perception.[4]
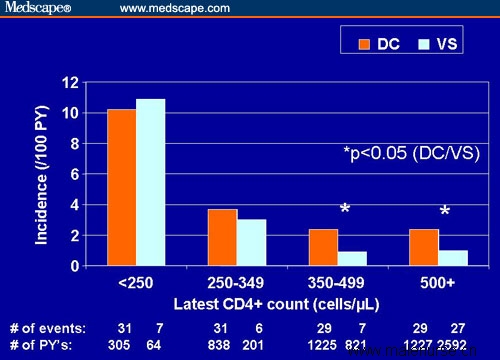
Figure 4. Incidence of opportunistic disease or death by latest CD4+ cell count stratum in the DC vs VS study arms. Adapted from Lundgren et al[3] with permission.
The findings of the SMART study were further supported by the results of the DART study,[5] which used a defined period in the STI arm, with 12 weeks on ART followed by 12 weeks off ART. The study randomized 813 patients to the STI or continuous therapy arm. Although there was no difference in the number of deaths during the median follow-up period of 51 weeks, the hazard ratio for clinical World Health Organization (WHO) stage 4 events was 2.6 for the STI arm, and the study investigators concluded that the STI strategy cannot be recommended. In contrast, the results of the BASTA study, reported by Maggiolo and colleagues,[6] appeared to support the use of STI, at least in selected patients. Although this study was done quite carefully, there were 76 patients in the STI arm and 38 in the continued HAART (highly active antiretroviral therapy) arm. In contrast to the statistical power of the SMART study, with over 5000 patients, this study was very underpowered and the results could happen by chance.
At this time, the overall conclusion is that the strategy of treatment interruptions cannot be recommended. In the SMART study, no subgroups could be found that benefited from such interruptions. Although further thought will be given to the underlying reasons for the results observed to date, neither CD4-guided nor time-defined STI has a place in clinical practice.
Initial Therapy
HAART in the DART Study
In keeping with the fact that this is truly an international conference with the theme "Time to Deliver," Munderi and coworkers[7] presented data on the 2-year survival and causes of death among patients (n = 1819) enrolled in the Entebbe, Uganda, cohort of the Development of ART in Africa (DART) trial. Survival and other clinical parameters of patients in the DART cohort were compared with those of a matched pre-ART historical cohort (n = 658). Patients in the DART cohort were randomized to receive ART consisting of coformulated zidovudine/lamivudine + efavirenz or coformulated zidovudine/lamivudine + tenofovir. The median CD4+ cell count at enrollment was 93 cells/mcL in the DART cohort and 75 cells/mcL in the historical controls. Approximately one third of patients in each cohort had CD4+ cells < 50/mcL. Despite the low CD4+ cell counts, there was a dramatic effect in the DART cohort (Figure 5). Overall, there was a 17-fold greater risk for death in the pre-ART group, and the benefit was even seen in the patients with very low CD4+ cell counts, in whom there was a relative risk of 16 for patients in the pre-ART period.
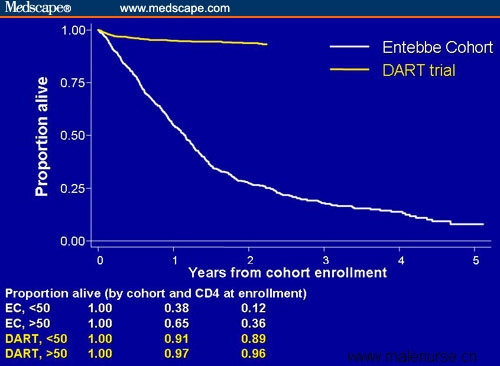
Figure 5. Survival in patients in the Entebbe DART cohort who received ART vs a historical untreated cohort.
Head-to-Head Comparison of 2 Boosted PIs
The KLEAN study was a large, multinational, phase 3b, open-label, 48-week analysis of 2 boosted protease inhibitor (PI) regimens: fosamprenavir/ritonavir (700/100 mg twice daily; fosAPV/r) vs lopinavir/ritonavir (400/100 mg twice daily; LPV/r) combined with fixed-dose abacavir/lamivudine given once daily. Data were presented in the late-breaker session, and the study results were published concurrently in The Lancet.[8,9]
A total of 878 antiretroviral-naive subjects were randomized 1:1 to fosAPV (n = 434) or LPV (n = 444), and were stratified at entry by HIV RNA above or below 100,000 copies/mL. Protocol-defined virologic failure was reduction of HIV RNA to < 400 copies/mL with subsequent increase to ≥ 400 copies/mL on 2 separate measurements, or failure to achieve HIV RNA < 400 copies/mL by week 24.
Baseline demographics for the study were similar across both arms. Median age was 38 years, median HIV RNA was 5.07 log10 copies/mL, and median CD4+ cell count was 192 cells/mcL. Twenty-two percent of subjects were women, 42% were non-white, and 15% of subjects were coinfected with hepatitis B virus (HBV), hepatitis C virus (HCV), or both HBV and HCV.
Investigators discovered no significant differences between the 2 arms at week 48 in terms of percentage of patients with HIV RNA < 400 or < 50 copies/mL. Figure 6 shows the proportion of subjects with HIV RNA < 400 copies/mL at week 48, stratified by baseline HIV RNA and CD4+ cell count. No significant differences were observed in viral load measures between the 2 arms, nor for changes in CD4+ cell count from baseline. Furthermore, investigators detected no significant differences in the rate of grade 2-4 adverse events between the 2 arms, including fasting lipids and gastrointestinal disturbances.
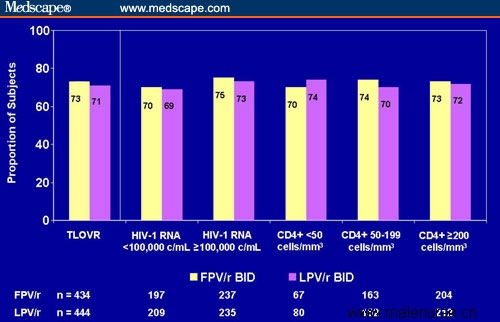
Figure 6. KLEAN study: proportion of subjects with HIV RNA < 400 copies/mL at week 48. Note: TLOVR = time to loss of virologic response. Value reflects all data for subjects on randomized PI. Responders defined as subjects with confirmed HIV RNA < 400 copies/mL who are not yet treatment failures. Treatment failures defined as subjects who do not achieve HIV RNA < 400 copies/mL, who have confirmed rebound after achieving this value, or who discontinue randomized PI for any reason. Published with permission of GlaxoSmithKline.
Conclusions. Although no significant differences were found between these 2 boosted-PI regimens, it is reassuring to know that patients have another boosted-PI option with efficacy and safety comparable to that of LPV/r. The recently published IAS-USA antiretroviral treatment guidelines[10] reflect this fact: 4 boosted PIs (atazanavir, fosamprenavir, lopinavir, and saquinavir) are recommended as first-line boosted-PI options, and abacavir/lamivudine (used as the nucleoside reverse transcriptase inhibitor [NRTI] backbone in this study) is recommended along with tenofovir/emtricitabine and zidovudine/lamivudine as a first-line NRTI option.
ARV Class Strategies
Although guidelines from various groups continue to offer recommendations for treatment-naive and -experienced patients, clinicians use their judgment to apply the best available information in order to individualize treatment. The information from clinical trials may lag behind what is actually practiced in a clinical setting, as discussed above regarding the KLEAN study. Although it is clear that both nonnucleoside reverse transcriptase inhibitor (NNRTI)- and PI-based HAART regimens are quite effective, the question as to whether they are equally effective for subgroups of patients has not been resolved.
CPCRA 058. The FIRST trial enrolled 1397 patients in a study that was designed to evaluate first-line therapy of NRTIs combined with: (1) a PI; (2) an NNRTI; or (3) both a PI and an NNRTI.[11] This trial followed patients for a median of 5 years. Because of the time of commencement several years ago of this planned long-term study, the predominant PI used was nelfinavir, and ritonavir boosting was rare. This makes it difficult to evaluate the results in the context of current clinical practice that uses boosted PIs. However, results showed that the NNRTI strategy was virologically superior to the PI strategy, but the 3-class strategy was not superior to either 2-class strategy. However, in the 5-year follow-up, there were no differences in CD4+ cell changes among the groups, making the outcome of undetectable viral load somewhat moot.
ACTG 5142. The ACTG 5142 trial was a strategy study that evaluated the relative efficacy of the 2 drug combinations that, as of August 24, 2006, still remain "preferred" according the DHHS guidelines: LPV/r+ 2 NRTIs vs efavirenz + 2 NRTIs. Each of these combinations "spares" one class of drugs, and a third arm evaluated LPV/r + EFV, a combination that "spared" NRTIs. Riddler and coinvestigators[12] presented the 96-week data from this trial in a late-breaker session at IAC (Table 1).
Table 1. Virologic and CD4+ Cell Results for 3 Class-Sparing Regimens: 96-Week Results From ACTG 5142
Outcome
LPV/r + EFV
LPV/r + NRTIs
EFV + NRTIs
Patients without virologic failure
(failure = HIV-RNA > 200 copies/mL after week 32), %
73
67
76
Patients with HIV RNA < 50 copies/mL, %
83
77*
89*
Median CD4+ cell increase, cells/mcL
268**
285**
239.5
*P = .003 for LPV/r + NRTIs vs EFV + NRTIs
**P = .01 for LPV/r + NRTIs and LPV/r + EFV vs EFV + NRTIs
EFV + NRTIs = efavirenz + nucleoside reverse transcriptase inhibitors; LPV/r = ritonavir-boosted lopinavir.
According to the results in this treatment-naive population who initiated treatment with a median viral load of 100,000 copies/mL and median CD4+ cell count slightly below 200 cells/mcL, virologic outcomes were comparable for the EFV + NRTIs and the EFV + LPV/r arms, but inferior in the LPV/r + NRTIs arm. There were substantial increases in CD4+ cell counts in all treatment arms, but the EFV + LPV/r and LPV/r + NRTIs arms outperformed the EFV + NRTIs arm with respect to this parameter.
The virologic outcomes were clearly superior in the EFV + NRTIs arm compared with the LPV/r + NRTIs arm. At present, these 2 options remain equally recommended in treatment guidelines. The results of this study, as well as the ACTG 5095 study discussed below, may cause that to be questioned. In addition, given the fact that the NNRTI + PI arm performed as well as the "top performer" with respect to both virologic and immunologic criteria, does this type of ART combination deserve a more favored place in our recommendations?
ACTG 5095. This ACTG study was designed to compare 3-drug treatment with ZDV + 3TC + EFV alone or combined with abacavir (ABC). The study enrolled 765 treatment-naive patients with a median baseline viral load of approximately 4.75 log10 and median CD4+ cell count of 213 in each study group. The recently published study results[13] showed that approximately 80% of patients in each study arm had HIV RNA < 50 copies/mL through 3 years of follow-up, and the conclusion was that there was no advantage to adding abacavir as the fourth drug.
Further analyses of the ACTG 5095 data resolve the ongoing concerns that EFV-based regimens are equally effective at all CD4+ cell counts and HIV RNA levels, and add to the data discussed above that this combination may be preferred as initial therapy. Ribaudo and coinvestigators[14] examined the long-term efficacy of ZDV + 3TC + EFV ± ABC in the 5095 study. This analysis stratified the patients by baseline viral load (copies/mL) into 4 categories: (1) ≥ 300,000; (2) 100,000-299,999; (3) 30,000-99,999; and (4) < 30,000. Virologic responses were also assessed by baseline CD4+ cell count (cells/mcL): (1) ≥ 500; (2) 350-499; (3) 200-349; (4) 50-199; and (5) < 50. Over a median follow-up of 3 years, neither pretreatment viral load (overall P = .63) nor baseline CD4+ cell count (P = .71) had a significant effect on virologic failure. These data confirm the efficacy of EFV-based regimens over a wide range of baseline viral loads and CD4+ cell counts.
Efficacy and Safety of Lopinavir/ritonavir as Monotherapy
Four separate trials presented at this year's IAC assessed the utility of LPV/r monotherapy. The rationale underlying these studies is the favorably high drug levels that are often observed with boosted PIs, including LPV/r, the potency of these agents, and their high genetic barrier to resistance. One of these studies evaluated the use of this boosted PI as initial therapy (MONARK), but the other 3, described below, assessed LPV/r as a maintenance strategy for patients with sustained virologic suppression on a triple-drug induction regimen.
MONARK trial. The Monotherapy Antiretroviral Kaletra (MONARK) trial[15] is a prospective, open-label pilot study comparing LPV/r monotherapy to a standard-of-care HAART regimen (LPV/r + zidovudine + lamivudine). Treatment-naive subjects with HIV RNA ≤ 100,000 copies/mL, CD4+ cell count ≥ 100 cells/mcL, and without evidence of resistance to study drugs were randomized to receive monotherapy (n = 83) or standard HAART (n = 53). Baseline characteristics between the 2 groups were similar. Primary endpoints were HIV RNA < 400 and < 50 copies/mL at weeks 24 and 48, respectively.
As reported by Delfraissy and colleagues,[15] intent-to-treat and on-treatment analyses for the 2 primary endpoints are shown in Table 2. No differences in tolerability were noted.
Table 2. MONARK Trial: LPV/r vs LPV/r + AZT + 3TC
Primary Endpoint
LPV/r,
n (%)
ITT
LPV/r +
AZT/3TC,
n (%)
ITT
LPV/r,
n (%)
OT
LPV/r +
AZT/3TC,
n (%)
OT
HIV RNA < 400 copies/mL, week 24
65/82
(79%)
41/53
(77%)
65/73
(89%)
41/42
(98%)
HIV RNA < 50 copies/mL, week 48
58/82
(71%)
40/53
(75%)
56/67
(84%)*
40/41
(98%)*
Both primary endpoints combined
(week 24 + week 48)
53/81
(65%)
40/53
(75%)
53/66
(80%)**
40/41
(98%)***P = .03; **P = .02
LPV/r = lopinavir/ritonavir; AZT = zidovudine; 3TC = lamivudine; ITT = intent-to-treat analysis; OT = on-treatment analysis.
From Delfraissy et al.[15] Published with permission.
The investigators concluded that although LPV/r did exert sustained virologic efficacy in this population, there were more patients randomized to LPV/r monotherapy who experienced low-level viremia, compared with those who were randomized to standard 3-drug HAART.
Study M03-613
In contrast to the MONARK study, 3 other trials assessed LPV/r after an induction period with standard 3-drug HAART. Cameron and coworkers[16] presented data from the M03-613 study in which 155 treatment-naive subjects were treated with LPV/r + zidovudine/lamivudine to "induce" viral loads < 50 copies/mL. After at least 24 weeks of treatment, and with 3 consecutive viral loads < 50 copies/mL, patients were randomized 2:1 to LPV/r monotherapy (n = 104) or to efavirenz + zidovudine/lamivudine (n = 51) as maintenance therapy.[16] The primary endpoint of the trial was percentage of patients who maintained HIV RNA < 50 copies/mL through week 96.
Baseline characteristics were similar between the arms, as were the percentages of subjects in each arm who experienced drug-related discontinuations. The primary analysis revealed that 50% and 61% of subjects in the LPV/r and efavirenz arms, respectively, achieved and maintained HIV RNA < 50 copies/mL (P = .23) (Figure 7). Investigators reported that most subjects in the LPV/r monotherapy arm who experienced blips between 50 and 500 copies/mL were able to resuppress viral load while on LPV/r monotherapy (11/12) or after restarting NRTIs with LPV/r (2/4). There was no significant difference observed in the percentage of PI or NNRTI mutations that developed in LPV/r- or efavirenz-treated subjects, respectively.
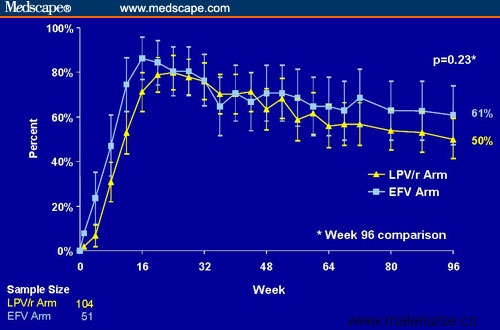
Figure 7. HIV-1 RNA < 50 copies/mL in patients randomized to maintenance therapy with LPV/r monotherapy or EFV/zidovudine/lamivudine after suppression to undetectable with LPV/r + zidovudine/lamivudine (intent-to-treat analysis, prior failure = failure). In this analysis using prior failure = failure, once patients met criteria for virologic failure (2 consecutive viral loads > 50 copies/mL), they were considered a virologic failure throughout the analysis, even if their viral load was subsequently resuppressed (with or without the readdition of NRTIs). The data on this slide represent all subjects randomized to the LPV/r arm vs the EFV arm. After 24 weeks of the study, most subjects in the LPV/r arm were receiving LPV/r monotherapy. By week 48, 95% of LPV/r patients were receiving LPV/r monotherapy. From Cameron et al.[16] Published with permission.
OK04 Study
Arribas and associates[17] presented results from another induction-maintenance trial using LPV/r. Patients who were receiving LPV/r + 2 NRTIs, and who had no history of virologic failure and HIV RNA <50 copies/mL for more than 6 months, were eligible for the study. At baseline, subjects were randomized to LPV/r or continuation of their triple-drug regimen. The primary endpoint was percentage of patients without virologic failure, defined as confirmed HIV RNA > 500 copies/mL, loss to follow-up, or change in randomized treatment.
A total of 198 subjects were randomized (monotherapy, n = 100; 3-drug continuation, n = 98), and baseline characteristics were similar across both arms. At week 48, 94% and 90% of subjects in the monotherapy and continuation arms, respectively, had not experienced protocol-defined treatment failure, although reasons for treatment failure in each arm were similar. Investigators reported that the 4 patients in the monotherapy arm with confirmed virologic rebound were reinduced with standard triple-drug therapy and have maintained HIV RNA < 50 copies/mL.
KalMo Study
Nunes and colleagues[18] presented interim, 48-week data from a 96-week, open-label, randomized trial assessing the utility of LPV/r monotherapy in patients with sustained virologic suppression with triple-drug therapy. Similar to the 2 trials above, eligible subjects had undetectable viral load for at least 6 months prior to entry and no evidence of prior virologic failure.
Sixty patients were enrolled, and baseline characteristics were similar between both arms of the study. By intention-to-treat analysis at week 48, 26/30 (87%) in the LPV/r monotherapy arm and 25/30 (83%) in the triple-drug control arm had HIV RNA < 80 copies/mL. One subject in each arm experienced HIV RNA > 1000 copies/mL, and there were no significant differences reported in terms of CD4+ cell counts, cholesterol, or anthropometric measures.
Conclusions
This collection of 4 studies shows that LPV/r exhibits less substantial virologic durability when compared with standard-of-care, triple-drug HAART regimens. The deficit of these boosted-PI monotherapy regimens compared with standard HAART is not dramatic, and it might not pose a substantial strategic danger in terms of long-term virologic outcome.
These monotherapy strategies seem to hold the greatest promise for patients who have already experienced sustained virologic suppression on a triple-drug induction regimen, and who then switch to the boosted PI as a maintenance regimen. However, more study is needed before this approach can be clinically recommended, and the take-home point from these 4 studies is that lopinavir monotherapy is consistently inferior, making it very difficult to justify using boosted-PI monotherapy.
ART Pharmacokinetics
Plasma Nevirapine Levels
As mentioned previously, an STI strategy has been studied in the DART study. In another analysis from this trial, reported by Kikaire and coworkers,[19] the pharmacokinetics of nevirapine (NVP) were studied after stopping NVP-based ART. There has been concern about stopping all of the ARVs in NNRTI-based regimens due to the long half-life and resistance profiles of NVP and EFV. In this study, NVP was stopped in 21 men and women, and either zidovudine-lamivudine or stavudine-lamivudine was continued to provide "coverage" during the vulnerable period of stopping NVP. A minimum inhibitory concentration of 200 ng/mL was considered to be the lower limit of the subtherapeutic range, with a therapeutic range of 3400-8000 ng/mL, and the "danger zone" lying between those values. After 7 days, 5 of 18 patients had NVP levels > 200 ng/mL, with levels below the limit of quantification (100 ng/mL) in 18 of 19 patients by week 2. The authors concluded that "these data support the use of a one-week dual nucleoside 'cover' for planned treatment interruptions."
The practice of continuing NRTIs when interrupting an NNRTI-based regimen to allow for the half-life of NVP is also applicable to EFV and to other drugs with similar resistance and pharmacokinetic profiles. It should be remembered that the NRTIs lamivudine and emtricitabine also have relatively long-half lives and a low genetic barrier to resistance.
Bioequivalence: New Triple-Drug, Fixed-Dose Pill
Shortly before the IAC, on July 12, 2006, the US Food and Drug Administration approved the first 1-pill, once-a-day treatment (Atripla; Bristol-Myers Squibb/Gilead Sciences) for HIV disease that had been long awaited. The components of this tablet have been approved as individual agents, and a coformulation of emtricitabine and tenofovir was also available. The addition of efavirenz to the mix proved challenging in terms of a viable formulation. At this meeting, data were presented that showed the bioequivalence of the coformulation to the individual drugs in healthy subjects, as shown below for the pharmacokinetic parameters of tenofovir (Table 3); results were similar for EFV and FTC.[20] Safety results were also similar: CNS adverse events were the most frequent treatment-emergent, drug-related adverse events — primarily dizziness and headache, occurring in 24% of subjects receiving the coformulated drug EFV/FTC/TDF and in 29% of subjects receiving EFV + FTC + TDF.
Table 3. Pharmacokinetics of Tenofovir in Healthy Subjects When Given as Coformulated Efavirenz(EFV)/Tenofovir(TDF)/Emtricitabine(FTC) or as Combinations of Individual Drugs (EFV + TDF + FTC)
PK Parameter
EFV/FTC/TDF
(Coformulated Pill)
EFV + FTC + TDF
(Separate Components)
% GMR
(90% CI)
Cmax (mcg/mL)
0.325 (34.2)
0.353 (29.6)
91.5 (84.6, 98.8)
AUC0-t (mcg.hr/mL)
1.95 (32.9)
1.97 (32.8)
99.3 (91.0, 108)
AUC0-∞ (mcg.hr/mL)
2.31 (29.2)
2.32 (30.3)
100 (93.2, 108)
T1/2 (hr)*
18.5 (7.70, 28.4)
17.2 (7.80, 31.4)
--* Median (min, max)
AUC0-t = Area-under-the plasma concentration time curve tau; AUC0-∞ = AUC from 1, extrapolated to infinity; CMax = maximum concentration of TFV; GMR = geometric least squares means; mcg = microgram; PK = pharmacokinetic; T1/2 = half-life
Darunavir for Treatment-Experienced Patients
The recent approval of darunavir has provided a new PI option for highly treatment-experienced patients. However, the long-term durability and safety remain important concerns that remain to be addressed. At this conference, 48-week efficacy and safety data of the pooled POWER 1 and 2 studies were presented.[21]
Patients were treated with the dose recommended for treatment-experienced patients: darunavir 600 mg with 100 mg ritonavir twice daily. Both the control-PI and darunavir regimens were administered with an optimized background regimen. At 48 weeks of follow-up, darunavir demonstrated continued activity; 61% (Table 4) maintained a viral load at least 1 log10 below baseline, compared with 70% at the 24-week follow-up time point. Diarrhea was the most common adverse event, occurring in 20% of patients (Table 5). The majority of adverse events were grade 1-2 in severity.
Table 4. 48-Week Efficacy Data From Pooled POWER 1 and POWER 2 Studies of Darunavir-Based vs Control Protease Inhibitors (CPI) in Treatment-Experienced Patients
Efficacy Parameter
Treatment Arm
P Value
Darunavir/ritonavir
(n = 110)
CPI
(n = 120)
≥ 1-log10 decrease in HIV-RNA
(% of patients)
61
15
< .001
HIV RNA < 50 copies/mL
(% of patients)
46
10
≤ .003
Mean HIV RNA reduction
(log10 copies/mL)
1.63
0.35
< .001
Mean CD4+ cell increase
(cells/microliter)
102
19
≤ .005
Table 5. 48-Week Adverse Events Reporting From Pooled POWER 1 and POWER 2 Studies of Darunavir-Based vs Control Protease Inhibitors (CPI) in Treatment-Experienced Patients
Adverse Event
Treatment Arm (% of Patients)
Darunavir/ritonavir
(n = 110)
CPI
(n = 120)
Diarrhea
20%
28%
Nausea
18%
13%
Headache
15%
20%
Nasopharyngitis
14%
11%
Fatigue
12%
17%
These data confirm the 24-week results observed with darunavir. More data are needed regarding the long-term efficacy and safety of this PI, as we have learned from tipranavir. In addition, we have learned time and time again that the greater the number of active drugs in a regimen, the greater and more durable the response. The need for new drugs in both new and existing classes of ARVs has not abated; fortunately, many promising investigational agents seem to be on the horizon.
References
1.El-Sadr W, Neaton J. Episodic CD4 guided use of antiretroviral therapy is inferior to continuous therapy: Results of the SMART study. Program and abstracts of the 13th Conference on Retroviruses and Opportunistic Infections; February 5-8, 2006; Denver, Colorado. Abstract 106LB.
2.El-Sadr W, for the SMART Study Group. Inferior clinical outcomes with episodic CD4-guided antiretroviral therapy aimed at drug conservation (DC) in SMART study: consistency of finding in all patient subgroups. Program and abstracts of the XVI International AIDS Conference; August 13-18, 2006; Toronto, Ontario, Canada. Abstract WEAB0204.
3.Lundgren JD, on behalf of the SMART Study Group. Progression of HIV-related disease or death (POD) in the randomised SMART Study: why was the risk of POD greater in the CD4-guided (re)-initiate ART at CD4 < 250 cells/microliter drug conservation (DC) vs the virological suppression (VS) arm? Program and abstracts of the XVI International AIDS Conference; August 13-18, 2006; Toronto, Ontario, Canada. Abstract WEAB0203.
4.Burman W, for the SMART Study Group. The effect of episodic CD4-guided antiretroviral therapy on quality of life: results of the quality of life substudy of SMART. Program and abstracts of the XVI International AIDS Conference; August 13-18, 2006; Toronto, Ontario, Canada. Abstract THPE0145.
5.Hakim J, on behalf of the DART trial team. A structured treatment interruption (STI) of 12 week cycles on and off ART is clinically inferior to continuous treatment in patients with low CD4 cell counts before ART: a randomisation within the DART trial. Program and abstracts of the XVI International AIDS Conference; August 13-18, 2006; Toronto, Ontario, Canada. Abstract THLB0207.
6.Maggiolo F, Ripamonti D, Callegaro A, et al. CD4-guided STI: four years follow-up of a controlled, prospective trial. Program and abstracts of the XVI International AIDS Conference; August 13-18, 2006; Toronto, Ontario, Canada. Abstract WEAB0202.
7.Munderi P, Watera C, Nakiyingi J, et al. Survival and causes of death, 2 years after introduction of antiretroviral therapy in Africa: a historical cohort comparison in Entebbe, Uganda. Program and abstracts of the XVI International AIDS Conference; August 13-18, 2006; Toronto, Ontario, Canada. Abstract THLB0208.
8.Eron J, Yeni P, Gathe J, et al. The KLEAN study: fosamprenavir + ritonavir (FPV/r) versus lopinavir/ritonavir (LPV/r) in antiretroviral-naive (ART-Naive) HIV-1 infected adults over 48 weeks. Program and abstracts of the XVI International AIDS Conference; August 13-18, 2006; Toronto, Ontario, Canada. Abstract THLB0205.
9.Eron J, Yeni P, Gathe J, et al. The KLEAN study of fosamprenavir-ritonavir versus lopinavir-ritonavir, each in combination with abacavir-lamivudine, for initial treatment of HIV infection over 48 weeks: a randomised non-inferiority trial. Lancet. 2006;368:476-482.
10.Hammer S, Saag M, Schechter M, et al. Treatment for adult HIV infection: 2006 recommendation of the International AIDS Society-USA Panel. JAMA. 2006;296:827-843.
11.MacArthur RD, Novak RM, Peng G, et al, for the CPCRA 058 Study Team and the Terry Beirn Community Programs for Clinical Research on AIDS (CPCRA). Long-term clinical and immunologic outcomes are similar in HIV-infected persons randomized to NNRTI vs PI vs NNRTI+PI-based antiretroviral regimens as initial therapy: results of the CPCRA 058 first study. Program and abstracts of the XVI International AIDS Conference; August 13-18, 2006; Toronto, Ontario, Canada. Abstract TUAB0102.
12.Riddler SA, Haubrich R, DiRienzo G, et al, for the AIDS Clinical Trials Group 5142 Study Team. A prospective, randomized, phase III trial of NRTI-, PI- and NNRTI-sparing regimens for initial treatment of HIV-1 infection-ACTG 5142. Program and abstracts of the XVI International AIDS Conference; August 13-18, 2006; Toronto, Ontario, Canada. Abstract THLB0204.
13.Gulick RM, Ribaudo HJ, Shikuma CM, et al, for the AIDS Clinical Trials Group (ACTG) A5095 Study Team. Three- vs four-drug antiretroviral regimens for the initial treatment of HIV-1 infection. JAMA. 2006;296:769-781.
14.Ribaudo H, Kuritzkes D, Lalama C, et al. Efavirenz (EFV)-based regimens are potent in treatment-naive subjects across a wide range of pre-treatment HIV-1 RNA (VL) and CD4 cell counts: 3-year results from ACTG 5095 (A5095). Program and abstracts of the XVI International AIDS Conference; August 13-18, 2006; Toronto, Ontario, Canada. Abstract THLB0211.
15.Delfraissy J-F, Flandre P, Delaugerre C, et al. MONARK trial (MONotherapy Antiretroviral Kaletra): 48-week analysis of lopinavir/ritonavir (LPV/r) monotherapy compared to LPV/r + zidovudine/lamivudine (AZT/3TC) in antiretroviral-naive patients. Program and abstracts of the XVI International AIDS Conference; August 13-18, 2006; Toronto, Ontario, Canada. Abstract THLB0202.
16.Cameron W, da Silva B, Arribas J, et al. A two-year randomized controlled clinical trial in antiretroviral-naive subjects using lopinavir/ritonavir (LPV/r) monotherapy after initial induction treatment compared to an efavirenz (EFV) 3-drug regimen (Study M03-613). Program and abstracts of the XVI International AIDS Conference; August 13-18, 2006; Toronto, Ontario, Canada. Abstract THLB0201.
17.Arribas J, Pulido F, Delgado R, et al, for the OK04 Study Group. Lopinavir/ritonavir as single-drug therapy in patients with HIV-1 viral suppression: forty eight week results of a randomized, controlled trial. Program and abstracts of the XVI International AIDS Conference; August 13-18, 2006; Toronto, Ontario, Canada. Abstract THLB0203.
18.Nunes EP, Oliviera MS, Almeida MMTB, et al. 48-week efficacy and safety results of simplification to single agent lopinavir/ritonavir (LPV/r) regimen in patients suppressed below 80 copies/mL on HAART — the KalMo study. Program and abstracts of the XVI International AIDS Conference; August 13-18, 2006; Toronto, Ontario, Canada. Abstract TUAB0103.
19.Kikaire B, Walker S, Khoo S, et al. Plasma levels of nevirapine following interruption of ZDV/3TC/NVP in African adults within the DART trial. Program and abstracts of the XVI International AIDS Conference; August 13-18, 2006; Toronto, Ontario, Canada. Abstract WEAB0201.
20.Mathias A, Plummer A, Skillington J, et al. Bioequivalence of the coformulation of efavirenz/emtricitabine/tenofovir DF. Program and abstracts of the XVI International AIDS Conference; August 13-18, 2006; Toronto, Ontario, Canada. Abstract TUE0098.
21.Lazzarin A, Queiroz-Telles F, Frank I, et al. TMC114 provides durable viral load suppression in treatment-experienced patients: POWER 1 and 2 combined week 48 analysis. Program and abstracts of the XVI International AIDS Conference; August 13-18, 2006; Toronto, Ontario, Canada. Abstract TUAB0104.
|

